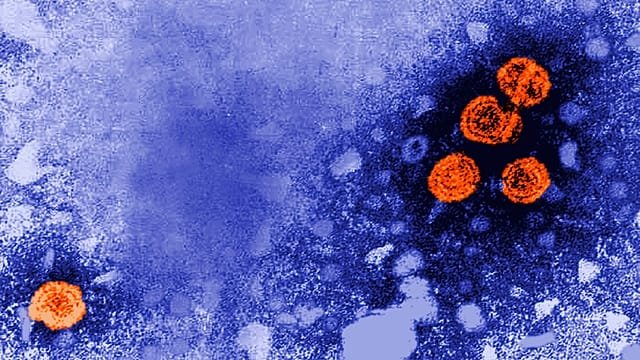
Guinea-Bissau has halted a US-backed hepatitis B vaccine study on newborns, pending an emergency ethical review.
The country’s health minister says a six-member ethics committee never met before the study was approved, a major red flag.
US-backed hepatitis B vaccine study halted in Guinea-Bissau
Tags
There’s no content to show here yet.
Latest Stories
- Singing contest in Rio prison offers creative outlet for women behind bars

- Ivory Coast’s Ouattara makes brother deputy PM in reshuffle

- YouTuber IShowSpeed rallies Benin on Africa tour

- Haiti’s crisis deepens after transitional council votes to oust prime minister

- IMF team to visit Gabon as authorities plan reform program

- France intercepts suspected ‘shadow fleet’ tanker from Russia

- Mozambique: crocodiles appear in towns amid floods

- Malawi launches cholera vaccine rollout

- Nigeria’s defense equipment from US to be delivered after five years

- United States officially exits the World Health Organization

Recent Comments
No comments to show.










Leave a Reply